what is the chlorination formula to treat water?
what is the general chlorination formula for powder form chlorine solution and liquid form chlorine solution?
3 Answers
Chlorine comes in many different forms, gas, liquid and solid. With a domestic tank the simplest form to use would be high volume chlorine tablets sufficient for the amount of water in the tank. It would also be possible to use High Test Hypochlorite granules (HTH) which are often used in swimming pools or a liquid domestic bleach or disinfectant. Each form has a different available chlorine content and it is useful to make up a standard 1 per cent chlorine solution (10g of chlorine/litre of water or 10,000mg/litre or 10,000 parts per million). This also ensures that solid forms of chlorine are fully dissolved before being added to the tank. The quantities of common forms of chlorine needed for a 1% solution are shown below.
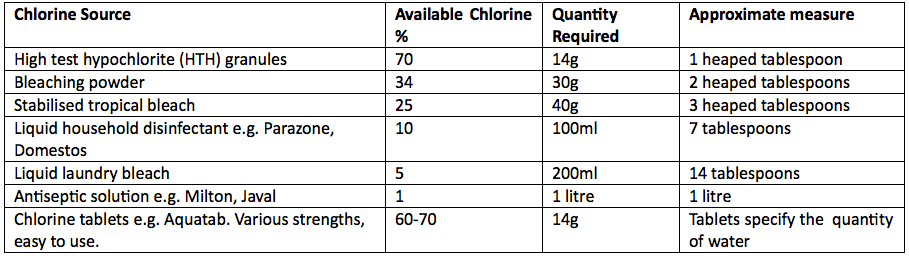
Table 1 – Quantities Required for a 1% Chlorine Solution
Enough chlorine must be provided to disinfect the water completely and to leave a free residual chlorine level in the water of 0.2-0.5mg/l. The chlorine residual level can be accurately measured with a simple colour comparator device but as a rough guide the free residual level is at the top of the right range when chlorine becomes detectable in the water by taste or smell. The dose of chlorine to add to achieve the desired free residual level depends on the quality of the water. If there is a high load of suspended solids, more chlorine will be needed. It is however usual that chlorine should be added in the range of 1–5 mg/litre. The amount of the 1% chlorine solution described above needed to achieve this range of chlorination are shown below.
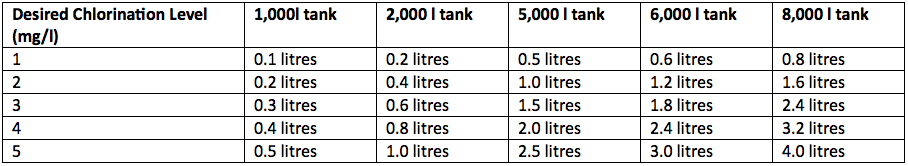
Table 2 – Quantity of 1% Chlorine Solution to add for Different Chlorination Levels
This chlorine should be allowed to run through the pipes so that they are also disinfected and then left to stand for at least 30 minutes as this contact time is necessary for it to kill all of the pathogens. Chlorine evaporates from a free water surface so water that is left in the tank will gradually reduce in chlorine content.
Chlorine powder is the common name for calcium hypochlorite, also known as bleaching powder or chloride of lime. Unfortunately it quality varies - commonly between 30-35% chlorine. Because it contains excess lime you want to make it up into a concentrated 'day tank' solution before dosing, this allows the lime to settle so you can draw the chlorine solution from the top.
In terms of formulae, try this from WHO (https://www.who.int/water_sanitation_h...):
W = 1000VC/S where: W = weight of powder required (grams) V = volume of tank (litres) C = concentration of solution required (% w/w) S = strength of powder (%chlorine w/w)
Commercially available chlorine solution is sodium hypochlorite. While chlorine powder decays with time, sodium hypochlorite's decay is much more rapid, so I definitely wouldn't trust what it says on the container. I would dose and measure, preferably with a closed control loop. Sodium Hypochlorite in concentrated form (10-15% w/w) is too concentrated to easily measure, hence after some dilution tests, I would recommend measuring in the treated water.
Here is the comparable WHO fact sheet for Sodium Hypochlorite: https://www.who.int/water_sanitation_h...
I imagine the above is what you mean by formula. But if you wanted the stoichiometry, the dissociation formula is the same for gas, powder, granules and solution; once chlorine has dissolved in water: https://www.aqueum.com/encyclopedia/ch...
Here are some resources about chlorination (particularly at the household level): https://resources.cawst.org/search/chlorine
This thread is public, all members of KnowledgePoint can read this page.







